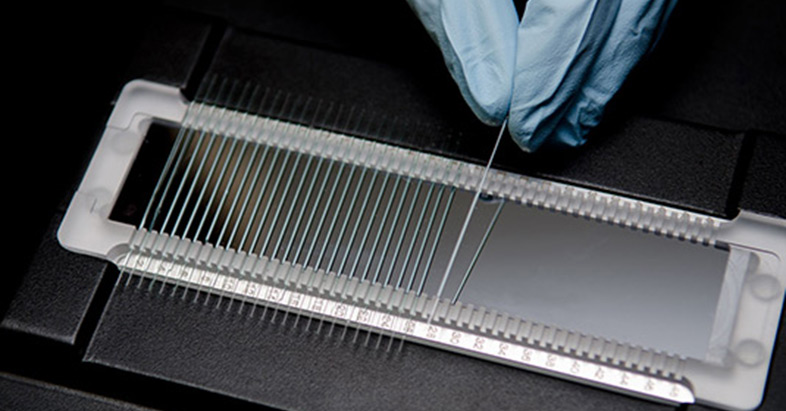
PR NT.48 用于检测蛋白稳定性。

PR NT.48 (PR001) + Backreflection Optics 用于检测蛋白稳定性和聚集情况。

Prometheus NT.48 (PR001) +背向反射光精准分析蛋白稳定性和聚集NanoTemper Technologies推出的Prometheus NT.48,采用nanoDSF技术,测量蛋白稳定性和聚集,具有超高分辨率。
主要特点:
背向反射光的主要特征:
Prometheus NT.48操作演示
"We quickly fell in love and made it the new workhorse in our lab"

DuPont Industrial Biosciences recently acquired its first Prometheus NT.48 nanoDSF instrument. We went through an extensive head to head comparison among other similar technologies and the nanoDSF stood out like a champion. We quickly fell in love and made it the new workhorse in our lab. The instrument provides great data quality of denaturation events with impressive signal to noise ratio, high density of data points, and extraordinary reproducibility making day to day analysis consistent and trustworthy. In addition, the instrument is straight forward to use and data very simple to analyze. We literally, got it, plugged it and started playing in no time.
We started employing the Prometheus to characterize protein samples in early stages of development as well as evaluating formulation stability of super concentrated protein solutions. The instrument is very versatile since measurements require very small sample volume, minimal effort in sample preparation and can handle relevant industrial protein concentrations of up to ~200 mg/mL. In this context, we appreciate the easy sample handling since samples are loaded directly from stock solutions into self-filling capillaries avoiding time consuming and complicated sample preparation steps, even for ultra-concentrated samples. Denaturation events missed by other DSF-type measurements, especially with super concentrated protein sample solutions, are clearly revealed through the high density of data points, yielding same data quality as the gold standard DSC.
Overall, we are very pleased and impressed with the many great qualities of the Prometheus. It will certainly allow us to bridge the gap between molecule screening, selection and product development. We are grateful to NanoTemper for creating this unique biophysical tool as well as for their outstanding and one of a kind customer service.
Dr. Mariliz Johnson, Senior Scientist at DuPont Industrial Biosciences, Palo Alto CA, USA
"The outstanding construction design allows for on-the-fly detection of fluorescence intensity resulting in impressive data point density"

In order for proteins to manifest their proper biological and therapeutic effect, their conformational and structural integrity must be maintained at all stages of the development and commercialization process. Thus, for the development of Biotherapeutics it is essential to find optimal (solution) conditions to stabilize the Drug Product such that we can ensure its safety, biological function, structural integrity, and storage for long term under unfavourable and favourable conditions.
To meet the requirements of current clinical indications with respect to drug delivery, highly concentrated therapeutic protein drugs up to 100-200 mg/ml are necessary. In order to achieve such concentrations, a number of protein properties like solubility, self-association, solution viscosity, and protein aggregation have to be controlled. The large dynamic range of the Prometheus NT.48 allows for analyzing thermal unfolding and re-folding in solutions containing protein concentrations between 250 mg/ml down to few µg/ml. Moreover, measurements with rather viscous sample solutions as sometimes observed by highly concentrated formulations can be performed. Thus, it can be utilized for both, stability screening during early phases of drug discovery where only small amounts of protein are available, as well as for formulation screening campaigns of highly concentrated samples. The capabilities of the Prometheus NT.48 itself and in combination with other complementary biophysical tools enables for a better understanding of conformational and colloidal properties of the protein candidate and their impact on macroscopic solution properties. However, in some cases, the Prometheus NT.48 allowed us to perform thermal stability screening with otherwise structurally very complex proteins which were virtually not amenable to any orthogonal biophysical technique.
Moreover, the Prometheus instrument allows for label-free analysis of 48 samples simultaneously independent of their protein concentrations (high dynamic range) and selected solution conditions and/or -compositions. Unlike other techniques the Prometheus NT.48 measurements remained unaffected by any excipient, sugar, detergent or additive. All together, the Prometheus instrument enables for very flexible experimental design and provides maintenance-free instrumentation. In addition, our obtained data demonstrate very high reproducibility, consistency, the robustness and precision of this particular technology. The outstanding construction design allows for on-the-fly detection of fluorescence intensity resulting in impressive data point density that there is virtually no need for data fitting.
I personally also highly appreciate the very intuitive software allowing for rapidly setting up the experiment and evaluation of obtained data. The software being developed allows for rapid/easy data analysis, data interpretation and exportation to other data processing software.
Dr. Michaela Blech, Associate Director, Global Pharmaceutical/Formulation Development, Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany
"We experienced that nanoDSF technology is superior to standard DSF"

Native, intrinsic fluorescence based nanoDSF technology has been quickly integrated in our standard operations and is applied nowadays in every project we are currently working on, including small molecules and biologics modalities. We are using Prometheus for initial screening as well as for lead optimization profiling of small molecules and fragments and for stability characterization of therapeutic proteins. Furthermore, nanoDSF is an invaluable tool for quality control since the fraction of unfolded protein can be quantified just within a few seconds.
The Prometheus NT.48 instrument is maintenance-free and provides an easy to use handling platform. The capillary format allows us to even measure highly viscous formulation conditions.
We experienced that nanoDSF technology is superior to standard DSF regarding its application range as well as in terms of precision, reproducibility, wider applicability and greater potential for new applications development.
Dr. Alexey Rak, Sanofi R&D, France


Lab Head Biophysical Characterization


With Targenomix being focused on target identification of small molecules, the protein quality is a major concern for us. As we work with a wide range of different protein species, the only way to provide premium data is to routinely test our protein samples for their stability and quality.
nanoDSF is the method of choice for our quality control procedure which we now apply as the very first step in every project since it allows very quick measurements and provides detailed information on sample properties.
The broad concentration range of the Prometheus NT.48 allows us for the first time to investigate protein stability at very different protein concentrations. Since proteins can both be stabilized or destabilized with increasing protein concentrations, this analysis is crucial for the development of the experimental conditions for further binding assays, such as MST. Moreover, nanoDSF provides us key parameters for optimal handling and storage conditions. Related to this, we appreciate the high reproducibility and resolution of the unfolding data obtained with the Prometheus NT.48.
"We quickly fell in love and made it the new workhorse in our lab"

DuPont Industrial Biosciences recently acquired its first Prometheus NT.48 nanoDSF instrument. We went through an extensive head to head comparison among other similar technologies and the nanoDSF stood out like a champion. We quickly fell in love and made it the new workhorse in our lab. The instrument provides great data quality of denaturation events with impressive signal to noise ratio, high density of data points, and extraordinary reproducibility making day to day analysis consistent and trustworthy. In addition, the instrument is straight forward to use and data very simple to analyze. We literally, got it, plugged it and started playing in no time.
We started employing the Prometheus to characterize protein samples in early stages of development as well as evaluating formulation stability of super concentrated protein solutions. The instrument is very versatile since measurements require very small sample volume, minimal effort in sample preparation and can handle relevant industrial protein concentrations of up to ~200 mg/mL. In this context, we appreciate the easy sample handling since samples are loaded directly from stock solutions into self-filling capillaries avoiding time consuming and complicated sample preparation steps, even for ultra-concentrated samples. Denaturation events missed by other DSF-type measurements, especially with super concentrated protein sample solutions, are clearly revealed through the high density of data points, yielding same data quality as the gold standard DSC.
Overall, we are very pleased and impressed with the many great qualities of the Prometheus. It will certainly allow us to bridge the gap between molecule screening, selection and product development. We are grateful to NanoTemper for creating this unique biophysical tool as well as for their outstanding and one of a kind customer service.
Dr. Mariliz Johnson, Senior Scientist at DuPont Industrial Biosciences, Palo Alto CA, USA
"The outstanding construction design allows for on-the-fly detection of fluorescence intensity resulting in impressive data point density"

In order for proteins to manifest their proper biological and therapeutic effect, their conformational and structural integrity must be maintained at all stages of the development and commercialization process. Thus, for the development of Biotherapeutics it is essential to find optimal (solution) conditions to stabilize the Drug Product such that we can ensure its safety, biological function, structural integrity, and storage for long term under unfavourable and favourable conditions.
To meet the requirements of current clinical indications with respect to drug delivery, highly concentrated therapeutic protein drugs up to 100-200 mg/ml are necessary. In order to achieve such concentrations, a number of protein properties like solubility, self-association, solution viscosity, and protein aggregation have to be controlled. The large dynamic range of the Prometheus NT.48 allows for analyzing thermal unfolding and re-folding in solutions containing protein concentrations between 250 mg/ml down to few µg/ml. Moreover, measurements with rather viscous sample solutions as sometimes observed by highly concentrated formulations can be performed. Thus, it can be utilized for both, stability screening during early phases of drug discovery where only small amounts of protein are available, as well as for formulation screening campaigns of highly concentrated samples. The capabilities of the Prometheus NT.48 itself and in combination with other complementary biophysical tools enables for a better understanding of conformational and colloidal properties of the protein candidate and their impact on macroscopic solution properties. However, in some cases, the Prometheus NT.48 allowed us to perform thermal stability screening with otherwise structurally very complex proteins which were virtually not amenable to any orthogonal biophysical technique.
Moreover, the Prometheus instrument allows for label-free analysis of 48 samples simultaneously independent of their protein concentrations (high dynamic range) and selected solution conditions and/or -compositions. Unlike other techniques the Prometheus NT.48 measurements remained unaffected by any excipient, sugar, detergent or additive. All together, the Prometheus instrument enables for very flexible experimental design and provides maintenance-free instrumentation. In addition, our obtained data demonstrate very high reproducibility, consistency, the robustness and precision of this particular technology. The outstanding construction design allows for on-the-fly detection of fluorescence intensity resulting in impressive data point density that there is virtually no need for data fitting.
I personally also highly appreciate the very intuitive software allowing for rapidly setting up the experiment and evaluation of obtained data. The software being developed allows for rapid/easy data analysis, data interpretation and exportation to other data processing software.
Dr. Michaela Blech, Associate Director, Global Pharmaceutical/Formulation Development, Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany
"We experienced that nanoDSF technology is superior to standard DSF"

Native, intrinsic fluorescence based nanoDSF technology has been quickly integrated in our standard operations and is applied nowadays in every project we are currently working on, including small molecules and biologics modalities. We are using Prometheus for initial screening as well as for lead optimization profiling of small molecules and fragments and for stability characterization of therapeutic proteins. Furthermore, nanoDSF is an invaluable tool for quality control since the fraction of unfolded protein can be quantified just within a few seconds.
The Prometheus NT.48 instrument is maintenance-free and provides an easy to use handling platform. The capillary format allows us to even measure highly viscous formulation conditions.
We experienced that nanoDSF technology is superior to standard DSF regarding its application range as well as in terms of precision, reproducibility, wider applicability and greater potential for new applications development.
Dr. Alexey Rak, Sanofi R&D, France
Whitepaper by ChromoTek GmbH
Use of iFormulate™ and nanoDSF for Fast and Precise Protein Formulation Development
One of the challenges in protein formulation is the simultaneous evaluation of multiple key formulation variables in a rational fashion. The Design of Experiments (DOE) approach has gained significant popularity in protein formulation as well as other process development activities.
HTD Biosystems has developed a robust DOE approach for protein formulations referred to as iFormulate™ that utilizes advanced response surface quadratic modeling to evaluate the effects of four formulation variables in a multivariate fashion. The procedure allows for rapid identification of stable protein formulations, using only small amounts of material.
By analyzing the thermal stability of a model protein, Lysozyme, using the Prometheus NT.48 in conjunction with the iFormulate™ DOE approach, we demonstrate that ideal formulations can be identified in as little as 35 minutes with minimal sample consumption. The DOE analysis of the precise and reproducible data generated by NanoTemper Technologies Prometheus NT.48 from 25 trial formulations resulted in the identification of the formulation design space for lysozyme formulations with high melting temperatures (Tms). The formulations can be rationalized using Quality by Design principles from the DOE analysis. Thus, by using the predictions from the DOE output and high-precision thermal stability analysis by nanoDSF, stable formulations of proteins can be generated and validated with unprecedented speed and precision.
nanoDSF Thermal Unfolding Analysis of a Membrane-bound Esterase in Various Detergents
Whitepaper by ChromoTek GmbH
Use of iFormulate™ and nanoDSF for Fast and Precise Protein Formulation Development
One of the challenges in protein formulation is the simultaneous evaluation of multiple key formulation variables in a rational fashion. The Design of Experiments (DOE) approach has gained significant popularity in protein formulation as well as other process development activities.
HTD Biosystems has developed a robust DOE approach for protein formulations referred to as iFormulate™ that utilizes advanced response surface quadratic modeling to evaluate the effects of four formulation variables in a multivariate fashion. The procedure allows for rapid identification of stable protein formulations, using only small amounts of material.
By analyzing the thermal stability of a model protein, Lysozyme, using the Prometheus NT.48 in conjunction with the iFormulate™ DOE approach, we demonstrate that ideal formulations can be identified in as little as 35 minutes with minimal sample consumption. The DOE analysis of the precise and reproducible data generated by NanoTemper Technologies Prometheus NT.48 from 25 trial formulations resulted in the identification of the formulation design space for lysozyme formulations with high melting temperatures (Tms). The formulations can be rationalized using Quality by Design principles from the DOE analysis. Thus, by using the predictions from the DOE output and high-precision thermal stability analysis by nanoDSF, stable formulations of proteins can be generated and validated with unprecedented speed and precision.
nanoDSF Thermal Unfolding Analysis of a Membrane-bound Esterase in Various Detergents
Orthogonal Methods for Characterizing the Unfolding of Therapeutic Monoclonal Antibodies: Differential Scanning Calorimetry, Isothermal Chemical Denaturation, and Intrinsic Fluorescence with Concomitant Static Light Scattering
Methods in Enzymology
Deniz B. Temel, Pavel Landsman, Mark L. Brader
Abstract
Evaluating prospective protein pharmaceutical stability from accelerated screening is a critical challenge in biotherapeutic discovery and development. Measurements of protein unfolding transitions are widely employed for comparing candidate molecules and formulations; however, the interrelationships between intrinsic protein conformational stability and pharmaceutical robustness are complex and thermal unfolding measurements can be misleading. Beyond the discovery phase of drug development, astute formulation design is one of the most crucial factors enabling the protein to resist damage to its higher order structure-initially from bioprocessing stresses, then from stresses encountered during its journey from the product manufacturing site to the bloodstream of the patient. Therapeutic monoclonal antibodies are multidomain proteins that represent a large and growing segment of the biotechnology pipeline. In this chapter, we describe how differential scanning calorimetry may be leveraged synergistically with isothermal chemical denaturation and intrinsic fluorescence with concomitant static light scattering to elucidate characteristics of mAb unfolding and aggregation that are helpful toward understanding and designing optimal pharmaceutical compositions for these molecules.
Novel microscale approaches for easy, rapid determination of protein stability in academic and commercial settings
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics
Crispin G. Alexander, Randy Wanner, Christopher M. Johnson, Dennis Breitsprecher, Gerhard Winter, Stefan Duhr, Philipp Baaske, Neil Ferguson
Abstract
Chemical denaturant titrations can be used to accurately determine protein stability. However, data acquisition is typically labour intensive, has low throughput and is difficult to automate. These factors, combined with high protein consumption, have limited the adoption of chemical denaturant titrations in commercial settings. Thermal denaturation assays can be automated, sometimes with very high throughput. However, thermal denaturation assays are incompatible with proteins that aggregate at high temperatures and large extrapolation of stability parameters to physiological temperatures can introduce significant uncertainties. We used capillary-based instruments to measure chemical denaturant titrations by intrinsic fluorescence and microscale thermophoresis. This allowed higher throughput, consumed several hundred-fold less protein than conventional, cuvette-based methods yet maintained the high quality of the conventional approaches. We also established efficient strategies for automated, direct determination of protein stability at a range of temperatures via chemical denaturation, which has utility for characterising stability for proteins that are difficult to purify in high yield. This approach may also have merit for proteins that irreversibly denature or aggregate in classical thermal denaturation assays. We also developed procedures for affinity ranking of protein-ligand interactions from ligand-induced changes in chemical denaturation data, and proved the principle for this by correctly ranking the affinity of previously unreported peptide-PDZ domain interactions. The increased throughput, automation and low protein consumption of protein stability determinations afforded by using capillary-based methods to measure denaturant titrations, can help to revolutionise protein research. We believe that the strategies reported are likely to find wide applications in academia, biotherapeutic formulation and drug discovery programmes.
Keywords
nanoDSF, Chemical denaturation, Thermal denaturation
nanoDSF reveals how HI virus acquires genetic material and how it can be stopped
The human immunodeficiency virus (HIV) is responsible for the acquired immune deficiency syndrome (AIDS). While there is currently no cure for HIV/AIDS, combinations of drugs are currently used as treatment to control the viral spread either by blocking its entrance to cells or by prohibiting the virus from making copies of itself. However, a study published in the May 30 issue of Nature, took a closer look to the structure of HIV's protein shell, also known as the capsid, revealing previously unknown structural and functional features that could be targeted as a powerful alternative to current HIV therapies.
With the combination of atomic-level structural work, virology and nanoDSF, new structural details revealed that there are strongly positively charged pores in the capsid, through which nucleotides are imported at great speed to allow the viral genome to be made. These size selective pores open and close keeping out any unwanted molecules, explaining why HIV is so successful at evading the immune system.
Moreover, it was shown that by blocking the capsid pore by a small molecule, HIV reverse transcription was inhibited, leaving the HI virus unable to copy itself and thus becoming non-infectious.
This capsid feature may be common to other viruses and will be an attractive target for new antiviral drugs.
We are excited that nanoDSF played an important role in this discovery!
We care about your research
Failure of ubiquitinating signaling system identified as the cause of fatal autoinmune disease
In society, failure in communication can lead to conflicts in many ways. Similarly, within an organism context, failure in communication between and within cells can cause many diseases. Among the mechanisms to establish precise cellular communication is ubiquitination, a post-translational modification with the highly conserved 76-residue small protein ubiquitin. A classic example of signaling through this ubiquitin 'tag' is the targeting of obsolete proteins for degradation in the proteosome. But it also plays important roles in regulating the immune system, where it controls transcription factors that orchestrate immune resposes.
In August 25 issue of Cell, the cause of a potentially fatal inherited autoimmune disease was identified for the first time involving a dysfunction of the ubiquitin system. In this disease, now named OTULIN-related autoinflammatory syndrome (ORAS), the immune system of patients spontaneously activates and start attacking the patient's own body shortly after birth, eventually leading to the child's death. The researchers of this study showed that the disease is caused by the simple substitution of a Leucine for a Proline in the deubiquitinating enzyme OTULIN, which acts as a brake preventing the immune system to take off without permission. Without this enzyme, the levels of linear ubiquitin is uncontrolled leading to a hyperactivated immune response even in the absence of infection.
In this work, nanoDSF played a crucial role in the analysis and comparison of stability and activity between normal and disease-causing mutated OTULIN. Moreover, this breakthrough discovered a treatment for ORAS, with an antibody that blocks TNF, one of the primary molecules that mediate the activation of the immune system. Luckily this antibody drug (Infliximab or Remicade) is already approved for the treatment of other autoinmune and inflammatory diseases making it easily accesible to patients and a repurposing of drugs.
We are proud to see that nanoDSF was applied in this great study.
We care about your research
DNA-binding proteins from marine bacteria expand the known sequence diversity of TALE-like repeats
Nucleic Acids Research
Orlando de Lange, Christina Wolf, Philipp Thiel, Jens Krüger, Christian Kleusch,Oliver Kohlbacher, Thomas Lahaye
Abstract
Transcription Activator-Like Effectors (TALEs) of Xanthomonas bacteria are programmable DNA binding proteins with unprecedented target specificity. Comparative studies into TALE repeat structure and function are hindered by the limited sequence variation among TALE repeats. More sequence-diverse TALE-like proteins are known from Ralstonia solanacearum (RipTALs) and Burkholderia rhizoxinica (Bats), but RipTAL and Bat repeats are conserved with those of TALEs around the DNA-binding residue. We study two novel marine-organism TALE-like proteins (MOrTL1 and MOrTL2), the first to date of non-terrestrial origin. We have assessed their DNA-binding properties and modelled repeat structures. We found that repeats from these proteins mediate sequence specific DNA binding conforming to the TALE code, despite low sequence similarity to TALE repeats, and with novel residues around the BSR. However, MOrTL1 repeats show greater sequence discriminating power than MOrTL2 repeats. Sequence alignments show that there are only three residues conserved between repeats of all TALE-like proteins including the two new additions. This conserved motif could prove useful as an identifier for future TALE-likes. Additionally, comparing MOrTL repeats with those of other TALE-likes suggests a common evolutionary origin for the TALEs, RipTALs and Bats.
Keywords
Monolith NT.115, Protein-Nucleic Acids, Biomolecular Interaction, Prometheus NT.48, Protein Stability
Orthogonal Methods for Characterizing the Unfolding of Therapeutic Monoclonal Antibodies: Differential Scanning Calorimetry, Isothermal Chemical Denaturation, and Intrinsic Fluorescence with Concomitant Static Light Scattering
Methods in Enzymology
Deniz B. Temel, Pavel Landsman, Mark L. Brader
Abstract
Evaluating prospective protein pharmaceutical stability from accelerated screening is a critical challenge in biotherapeutic discovery and development. Measurements of protein unfolding transitions are widely employed for comparing candidate molecules and formulations; however, the interrelationships between intrinsic protein conformational stability and pharmaceutical robustness are complex and thermal unfolding measurements can be misleading. Beyond the discovery phase of drug development, astute formulation design is one of the most crucial factors enabling the protein to resist damage to its higher order structure-initially from bioprocessing stresses, then from stresses encountered during its journey from the product manufacturing site to the bloodstream of the patient. Therapeutic monoclonal antibodies are multidomain proteins that represent a large and growing segment of the biotechnology pipeline. In this chapter, we describe how differential scanning calorimetry may be leveraged synergistically with isothermal chemical denaturation and intrinsic fluorescence with concomitant static light scattering to elucidate characteristics of mAb unfolding and aggregation that are helpful toward understanding and designing optimal pharmaceutical compositions for these molecules.
Novel microscale approaches for easy, rapid determination of protein stability in academic and commercial settings
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics
Crispin G. Alexander, Randy Wanner, Christopher M. Johnson, Dennis Breitsprecher, Gerhard Winter, Stefan Duhr, Philipp Baaske, Neil Ferguson
Abstract
Chemical denaturant titrations can be used to accurately determine protein stability. However, data acquisition is typically labour intensive, has low throughput and is difficult to automate. These factors, combined with high protein consumption, have limited the adoption of chemical denaturant titrations in commercial settings. Thermal denaturation assays can be automated, sometimes with very high throughput. However, thermal denaturation assays are incompatible with proteins that aggregate at high temperatures and large extrapolation of stability parameters to physiological temperatures can introduce significant uncertainties. We used capillary-based instruments to measure chemical denaturant titrations by intrinsic fluorescence and microscale thermophoresis. This allowed higher throughput, consumed several hundred-fold less protein than conventional, cuvette-based methods yet maintained the high quality of the conventional approaches. We also established efficient strategies for automated, direct determination of protein stability at a range of temperatures via chemical denaturation, which has utility for characterising stability for proteins that are difficult to purify in high yield. This approach may also have merit for proteins that irreversibly denature or aggregate in classical thermal denaturation assays. We also developed procedures for affinity ranking of protein-ligand interactions from ligand-induced changes in chemical denaturation data, and proved the principle for this by correctly ranking the affinity of previously unreported peptide-PDZ domain interactions. The increased throughput, automation and low protein consumption of protein stability determinations afforded by using capillary-based methods to measure denaturant titrations, can help to revolutionise protein research. We believe that the strategies reported are likely to find wide applications in academia, biotherapeutic formulation and drug discovery programmes.
Keywords
nanoDSF, Chemical denaturation, Thermal denaturation
nanoDSF reveals how HI virus acquires genetic material and how it can be stopped
The human immunodeficiency virus (HIV) is responsible for the acquired immune deficiency syndrome (AIDS). While there is currently no cure for HIV/AIDS, combinations of drugs are currently used as treatment to control the viral spread either by blocking its entrance to cells or by prohibiting the virus from making copies of itself. However, a study published in the May 30 issue of Nature, took a closer look to the structure of HIV's protein shell, also known as the capsid, revealing previously unknown structural and functional features that could be targeted as a powerful alternative to current HIV therapies.
With the combination of atomic-level structural work, virology and nanoDSF, new structural details revealed that there are strongly positively charged pores in the capsid, through which nucleotides are imported at great speed to allow the viral genome to be made. These size selective pores open and close keeping out any unwanted molecules, explaining why HIV is so successful at evading the immune system.
Moreover, it was shown that by blocking the capsid pore by a small molecule, HIV reverse transcription was inhibited, leaving the HI virus unable to copy itself and thus becoming non-infectious.
This capsid feature may be common to other viruses and will be an attractive target for new antiviral drugs.
We are excited that nanoDSF played an important role in this discovery!
We care about your research
Failure of ubiquitinating signaling system identified as the cause of fatal autoinmune disease
In society, failure in communication can lead to conflicts in many ways. Similarly, within an organism context, failure in communication between and within cells can cause many diseases. Among the mechanisms to establish precise cellular communication is ubiquitination, a post-translational modification with the highly conserved 76-residue small protein ubiquitin. A classic example of signaling through this ubiquitin 'tag' is the targeting of obsolete proteins for degradation in the proteosome. But it also plays important roles in regulating the immune system, where it controls transcription factors that orchestrate immune resposes.
In August 25 issue of Cell, the cause of a potentially fatal inherited autoimmune disease was identified for the first time involving a dysfunction of the ubiquitin system. In this disease, now named OTULIN-related autoinflammatory syndrome (ORAS), the immune system of patients spontaneously activates and start attacking the patient's own body shortly after birth, eventually leading to the child's death. The researchers of this study showed that the disease is caused by the simple substitution of a Leucine for a Proline in the deubiquitinating enzyme OTULIN, which acts as a brake preventing the immune system to take off without permission. Without this enzyme, the levels of linear ubiquitin is uncontrolled leading to a hyperactivated immune response even in the absence of infection.
In this work, nanoDSF played a crucial role in the analysis and comparison of stability and activity between normal and disease-causing mutated OTULIN. Moreover, this breakthrough discovered a treatment for ORAS, with an antibody that blocks TNF, one of the primary molecules that mediate the activation of the immune system. Luckily this antibody drug (Infliximab or Remicade) is already approved for the treatment of other autoinmune and inflammatory diseases making it easily accesible to patients and a repurposing of drugs.
We are proud to see that nanoDSF was applied in this great study.
We care about your research
DNA-binding proteins from marine bacteria expand the known sequence diversity of TALE-like repeats
Nucleic Acids Research
Orlando de Lange, Christina Wolf, Philipp Thiel, Jens Krüger, Christian Kleusch,Oliver Kohlbacher, Thomas Lahaye
Abstract
Transcription Activator-Like Effectors (TALEs) of Xanthomonas bacteria are programmable DNA binding proteins with unprecedented target specificity. Comparative studies into TALE repeat structure and function are hindered by the limited sequence variation among TALE repeats. More sequence-diverse TALE-like proteins are known from Ralstonia solanacearum (RipTALs) and Burkholderia rhizoxinica (Bats), but RipTAL and Bat repeats are conserved with those of TALEs around the DNA-binding residue. We study two novel marine-organism TALE-like proteins (MOrTL1 and MOrTL2), the first to date of non-terrestrial origin. We have assessed their DNA-binding properties and modelled repeat structures. We found that repeats from these proteins mediate sequence specific DNA binding conforming to the TALE code, despite low sequence similarity to TALE repeats, and with novel residues around the BSR. However, MOrTL1 repeats show greater sequence discriminating power than MOrTL2 repeats. Sequence alignments show that there are only three residues conserved between repeats of all TALE-like proteins including the two new additions. This conserved motif could prove useful as an identifier for future TALE-likes. Additionally, comparing MOrTL repeats with those of other TALE-likes suggests a common evolutionary origin for the TALEs, RipTALs and Bats.
Keywords
Monolith NT.115, Protein-Nucleic Acids, Biomolecular Interaction, Prometheus NT.48, Protein Stability