Monolith NT.Automated 是 MST 技术的集大成者

Monolith NT.自动化型
高通量版MST: Monolith NT.自动化型, 超值体验!
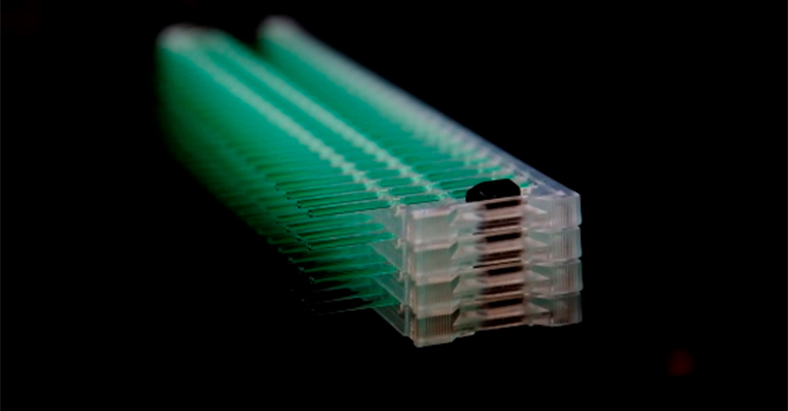
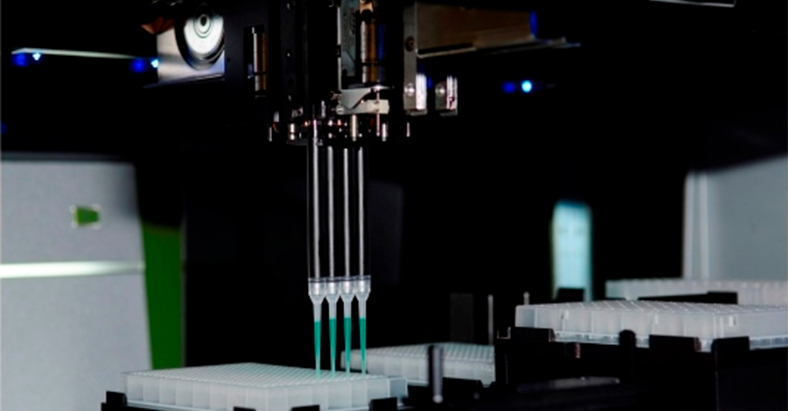
Monolith NT.自动化版利用微观热泳动技术,专为样品筛选自动化而设计。
Monolith NT.自动化型秉承了微量热泳动技术的所有优点,实验设置与 Monolith 系列相同。Monolith NT.自动化型可同时容纳96个样品,通过整合移液工作站(NT全自动样品筛选),样品处理时间缩短90%以上。
Monolith 的优点:
仅用16分钟,NT.自动化型就可以完成96个样品测试。Monolith NT.自动化型适用于不同的实验设计; 通过对96个样品的测定,可得出8条分别由12个数据点组成的结合曲线,也可以对96个样本进行单点筛选。
敬请参考此片段筛选应用实例。
最大通量的单数据点测试和解离常数测定:
Monolith 检测器
因为最多可配备两个不同的检测系统,在测试相互作用时,MonolithNT.自动化型有极强的多功能性。常见检测系统组合如下:










Monolith NT.Automated: Discover the fully automated MST performance
Monolith NT.Automated: Explore the ease-of-use for the standalone device
"It has proved a valuable tool for characterising small molecule-protein and protein-protein interactions"

"We routinely assess interaction affinity for both small molecule and biologics projects, with NanoTemper Technologies' Microscale Thermophoresis being the most recent addition to the pool of instruments we use to carry out these measurements. It has proved a valuable tool for characterising small molecule-protein and protein-protein interactions, as well as for the study of protein aggregation concentration determination. There is very good agreement with other technologies such as Surface Plasmon Resonance (SPR) and Isothermal Titration Calorimetry (ITC), and we are particularly appreciative of this new technology because of the extremely low protein consumption and relatively short time required for the assay setup. NanoTemper customer support has been a key factor in enabling us to familiarise ourselves with the new technology. We would like to deploy increasing numbers of applications based on MST technologies and continue to interact with NanoTemper Technologies Company, a dynamic, scientifically driven company."
Dr. Alexey Rak,
Head of Structural Biology & Biophysics, Sanofi R&D, France
"MST is a versatile and valuable tool which we quickly adapted in our repertoire of methods."

"One mission of the Structural Research group within the Lead Identification and Optimization department is to quantitatively validate and characterize interactions of small molecules as well as new biological entities with protein targets. We employ various traditional biophysical methods such as SPR, Thermal Shift, ITC, NMR and X-ray crystallography.
Most recently we included Microscale Thermophoresis (MST) in our standard project support workflow and extended its application from affinity determinations to fragment screening approaches. MST is a versatile and valuable tool which we quickly adapted in our repertoire of methods. The impressive advantages of MST, namely the low sample consumption, the broad application range, and swift assay development make it a unique biophysical method. The measurement in free solution without the need of surface coupling saves time and avoids a potential source for false positive or negative results.
Our Structural Research group now also added the label-free version of the Monolith to our MST instrumentation portfolio, which gives us the possibility to choose to measure with high selectivity and sensitivity (NT.115). The labelfree version (NT.LabelFree) allows us to measusre without any additional sample modification depending on the need of the particular assay. In some cases, LabelFree MST allowed us to perform assays with otherwise "very ill" behaved proteins which were not amenable to any other biophysical technique. Generally, we find very good consistency between quantitative MST measurements and results stemming from other biophysical methods."
Dr. Markus Zeeb, Principal Scientist, Structural Research, Boehringer-Ingelheim, Germany
"LabelFree MST is one of the few true label-free/immobilisation-free instruments capable of measuring molecular interactions"

"As part of our drug discovery projects we use Microscale Thermophoresis (MST) as an orthogonal method to measure the binding affinity of compounds to their protein target and apply this fragment hit ID through to lead optimisation. MST complements our biophysical platform and has correlated well with other more established technologies. Because it uses small amounts of protein, MST has proved to be particularly useful to look at molecular interaction involving proteins that are difficult to express or purify. MST requires a relatively short time to setup new assays and is a powerful technique for buffer optimisation. Using the NT.115 MST instrument we have successfully measured small molecule-protein and protein-protein interactions in complex media. Finally, LabelFree MST is one of the few true label-free/immobilisation-free instruments capable of measuring molecular interactions. We have appreciated the professionalism and support from NanoTemper Technologies."
Dr. Nicolas Basse, Department of Structural Biology, UCB-Celltech, UK
"MST data effectively “rescued” a hit list"

The European Lead Factory (ELF) is a very large academic/industrial collaboration aimed at providing European academics and Small and Medium-sized Enterprises (SMEs) access to industrial high throughput screening (HTS).
HTS yields many false positives and Pan-Assay Interference Compounds (PAINS) molecules which can be very difficult to triage out of the final hit list. Thus, in the ELF we use MicroScale Thermophoresis (MST) in some of our projects to validate target engagement. In this regard MST is a very valuable orthogonal biophysical approach not least because it is applicable to many different targets which are not suitable for SPR. There are a number of specific project examples whereby MST data effectively "rescued" a hit list and provided enough confidence for us to continue applying chemistry resources. These projects are ongoing and yielding very promising compounds.
Stuart McElroy, Head of Biology, Biological Chemistry and Drug Discovery, European Screening Centre (ESC), BoNess Road, Newhouse, Lanarkshire
"It has proved a valuable tool for characterising small molecule-protein and protein-protein interactions"

"We routinely assess interaction affinity for both small molecule and biologics projects, with NanoTemper Technologies' Microscale Thermophoresis being the most recent addition to the pool of instruments we use to carry out these measurements. It has proved a valuable tool for characterising small molecule-protein and protein-protein interactions, as well as for the study of protein aggregation concentration determination. There is very good agreement with other technologies such as Surface Plasmon Resonance (SPR) and Isothermal Titration Calorimetry (ITC), and we are particularly appreciative of this new technology because of the extremely low protein consumption and relatively short time required for the assay setup. NanoTemper customer support has been a key factor in enabling us to familiarise ourselves with the new technology. We would like to deploy increasing numbers of applications based on MST technologies and continue to interact with NanoTemper Technologies Company, a dynamic, scientifically driven company."
Dr. Alexey Rak,
Head of Structural Biology & Biophysics, Sanofi R&D, France
"MST is a versatile and valuable tool which we quickly adapted in our repertoire of methods."

"One mission of the Structural Research group within the Lead Identification and Optimization department is to quantitatively validate and characterize interactions of small molecules as well as new biological entities with protein targets. We employ various traditional biophysical methods such as SPR, Thermal Shift, ITC, NMR and X-ray crystallography.
Most recently we included Microscale Thermophoresis (MST) in our standard project support workflow and extended its application from affinity determinations to fragment screening approaches. MST is a versatile and valuable tool which we quickly adapted in our repertoire of methods. The impressive advantages of MST, namely the low sample consumption, the broad application range, and swift assay development make it a unique biophysical method. The measurement in free solution without the need of surface coupling saves time and avoids a potential source for false positive or negative results.
Our Structural Research group now also added the label-free version of the Monolith to our MST instrumentation portfolio, which gives us the possibility to choose to measure with high selectivity and sensitivity (NT.115). The labelfree version (NT.LabelFree) allows us to measusre without any additional sample modification depending on the need of the particular assay. In some cases, LabelFree MST allowed us to perform assays with otherwise "very ill" behaved proteins which were not amenable to any other biophysical technique. Generally, we find very good consistency between quantitative MST measurements and results stemming from other biophysical methods."
Dr. Markus Zeeb, Principal Scientist, Structural Research, Boehringer-Ingelheim, Germany
"LabelFree MST is one of the few true label-free/immobilisation-free instruments capable of measuring molecular interactions"

"As part of our drug discovery projects we use Microscale Thermophoresis (MST) as an orthogonal method to measure the binding affinity of compounds to their protein target and apply this fragment hit ID through to lead optimisation. MST complements our biophysical platform and has correlated well with other more established technologies. Because it uses small amounts of protein, MST has proved to be particularly useful to look at molecular interaction involving proteins that are difficult to express or purify. MST requires a relatively short time to setup new assays and is a powerful technique for buffer optimisation. Using the NT.115 MST instrument we have successfully measured small molecule-protein and protein-protein interactions in complex media. Finally, LabelFree MST is one of the few true label-free/immobilisation-free instruments capable of measuring molecular interactions. We have appreciated the professionalism and support from NanoTemper Technologies."
Dr. Nicolas Basse, Department of Structural Biology, UCB-Celltech, UK
"MST data effectively “rescued” a hit list"

The European Lead Factory (ELF) is a very large academic/industrial collaboration aimed at providing European academics and Small and Medium-sized Enterprises (SMEs) access to industrial high throughput screening (HTS).
HTS yields many false positives and Pan-Assay Interference Compounds (PAINS) molecules which can be very difficult to triage out of the final hit list. Thus, in the ELF we use MicroScale Thermophoresis (MST) in some of our projects to validate target engagement. In this regard MST is a very valuable orthogonal biophysical approach not least because it is applicable to many different targets which are not suitable for SPR. There are a number of specific project examples whereby MST data effectively "rescued" a hit list and provided enough confidence for us to continue applying chemistry resources. These projects are ongoing and yielding very promising compounds.
Stuart McElroy, Head of Biology, Biological Chemistry and Drug Discovery, European Screening Centre (ESC), BoNess Road, Newhouse, Lanarkshire
An Automated Microscale Thermophoresis Screening Approach for Fragment-Based Lead Discovery
Journal of Biomolecular Screening
Pawel Linke, Kwame Amaning, Melanie Maschberger, Francois Vallee, Valerie Steier, Philipp Baaske, Stefan Duhr, Dennis Breitsprecher, Alexey Rak
Abstract
Fragment-based lead discovery has proved to be an effective alternative to high-throughput screenings in identifying chemical matter that can be developed into robust lead compounds. The search for optimal combinations of biophysical techniques that can correctly and efficiently identify and quantify binding can be challenging due to the physicochemical properties of fragments. In order to minimize the time and costs of screening, optimal combinations of biophysical techniques with maximal information content, sensitivity, and robustness are needed. Here we describe an approach utilizing automated microscale thermophoresis (MST) affinity screening to identify fragments active against MEK1 kinase. MST identified multiple hits that were confirmed by X-ray crystallography but not detected by orthogonal methods. Furthermore, MST also provided information about ligand-induced aggregation and protein denaturation. The technique delivered a large number of binders while reducing experimentation time and sample consumption, demonstrating the potential of MST to execute and maximize the efficacy of fragment screening campaigns.
Keywords
Monolith NT.Automated, Protein-Fragment Interaction, Screening, Mulitple Component Interactions, Biomolecular Interaction
Discovery of Inter-Domain Stabilizers—A Novel Assay System for Allosteric Akt Inhibitors
ACS Chemical Biology
Zhizhou Fang, Jeffrey R. Simard, Dennis Plenker, Hoang D. Nguyen, Trang Phan, Patrik Wolle, Stefan Baumeister, Daniel Rauh
Abstract
In addition to the catalytically active kinase domain, most kinases feature regulatory domains that govern their activity. Modulating and interfering with these interdomain interactions presents a major opportunity for understanding biological systems and developing novel therapeutics. Therefore, small molecule inhibitors that target these interactions through an allosteric mode of action have high intrinsic selectivity, as these interactions are often unique to a single kinase or kinase family. Here we report the development of iFLiK (interface-Fluorescent Labels in Kinases), a fluorescence-based assay that can monitor such interdomain interactions. Using iFLiK, we have demonstrated selective detection of allosteric Akt inhibitors that induce an inactive closed conformation unique to Akt. This methodology easily distinguished small molecule allosteric inhibitors from classic ATP-competitive inhibitors. Screening an in-house compound library with iFLiK, we were able to identify novel compounds with a scaffold that has not been previously described for allosteric Akt inhibitors.
Keywords
Monolith NT.115; Protein-Small Molecule; Biomolecular Interaction
Receptor binding by a ferret-transmissible H5 avian influenza virus
Nature
Xiaoli Xiong, Peter J. Coombs, Stephen R. Martin, Junfeng Liu, Haixia Xiao, John W. McCauley, Kathrin Locher, Philip A. Walker, Patrick J. Collins, Yoshihiro Kawaoka, John J. Skehel & Steven J. Gamblin
Abstract
Cell-surface-receptor binding by influenza viruses is a key determinant of their transmissibility, both from avian and animal species to humans as well as from human to human. Highly pathogenic avian H5N1 viruses that are a threat to public health have been observed to acquire affinity for human receptors, and transmissible-mutant-selection experiments have identified a virus that is transmissible in ferrets1, 2, 3, the generally accepted experimental model for influenza in humans. Here, our quantitative biophysical measurements of the receptor-binding properties of haemagglutinin (HA) from the transmissible mutant indicate a small increase in affinity for human receptor and a marked decrease in affinity for avian receptor. From analysis of virus and HA binding data we have derived an algorithm that predicts virus avidity from the affinity of individual HA-receptor interactions. It reveals that the transmissible-mutant virus has a 200-fold preference for binding human over avian receptors. The crystal structure of the transmissible-mutant HA in complex with receptor analogues shows that it has acquired the ability to bind human receptor in the same folded-back conformation as seen for HA from the 1918, 1957 (ref. 4), 1968 (ref. 5) and 2009 (ref. 6) pandemic viruses. This binding mode is substantially different from that by which non-transmissible wild-type H5 virus HA binds human receptor. The structure of the complex also explains how the change in preference from avian to human receptors arises from the Gln226Leu substitution, which facilitates binding to human receptor but restricts binding to avian receptor. Both features probably contribute to the acquisition of transmissibility by this mutant virus.
Keywords
Animals; Birds/metabolism/virology; Chick Embryo; Crystallography, X-Ray; Ferrets/*virology; Hemagglutinin Glycoproteins; Influenza Virus/*chemistry/genetics/*metabolism; *Host Specificity;
Humans; Influenza A Virus; H5N1 Subtype/chemistry/*genetics/*metabolism/pathogenicity; Models, Biological; Models, Molecular; Mutation; Orthomyxoviridae Infections/*transmission/*virology; Protein Conformation; Receptors, Virus/*metabolism; Species Specificity; Monolith NT.115; Protein-Protein
An Automated Microscale Thermophoresis Screening Approach for Fragment-Based Lead Discovery
Journal of Biomolecular Screening
Pawel Linke, Kwame Amaning, Melanie Maschberger, Francois Vallee, Valerie Steier, Philipp Baaske, Stefan Duhr, Dennis Breitsprecher, Alexey Rak
Abstract
Fragment-based lead discovery has proved to be an effective alternative to high-throughput screenings in identifying chemical matter that can be developed into robust lead compounds. The search for optimal combinations of biophysical techniques that can correctly and efficiently identify and quantify binding can be challenging due to the physicochemical properties of fragments. In order to minimize the time and costs of screening, optimal combinations of biophysical techniques with maximal information content, sensitivity, and robustness are needed. Here we describe an approach utilizing automated microscale thermophoresis (MST) affinity screening to identify fragments active against MEK1 kinase. MST identified multiple hits that were confirmed by X-ray crystallography but not detected by orthogonal methods. Furthermore, MST also provided information about ligand-induced aggregation and protein denaturation. The technique delivered a large number of binders while reducing experimentation time and sample consumption, demonstrating the potential of MST to execute and maximize the efficacy of fragment screening campaigns.
Keywords
Monolith NT.Automated, Protein-Fragment Interaction, Screening, Mulitple Component Interactions, Biomolecular Interaction
Discovery of Inter-Domain Stabilizers—A Novel Assay System for Allosteric Akt Inhibitors
ACS Chemical Biology
Zhizhou Fang, Jeffrey R. Simard, Dennis Plenker, Hoang D. Nguyen, Trang Phan, Patrik Wolle, Stefan Baumeister, Daniel Rauh
Abstract
In addition to the catalytically active kinase domain, most kinases feature regulatory domains that govern their activity. Modulating and interfering with these interdomain interactions presents a major opportunity for understanding biological systems and developing novel therapeutics. Therefore, small molecule inhibitors that target these interactions through an allosteric mode of action have high intrinsic selectivity, as these interactions are often unique to a single kinase or kinase family. Here we report the development of iFLiK (interface-Fluorescent Labels in Kinases), a fluorescence-based assay that can monitor such interdomain interactions. Using iFLiK, we have demonstrated selective detection of allosteric Akt inhibitors that induce an inactive closed conformation unique to Akt. This methodology easily distinguished small molecule allosteric inhibitors from classic ATP-competitive inhibitors. Screening an in-house compound library with iFLiK, we were able to identify novel compounds with a scaffold that has not been previously described for allosteric Akt inhibitors.
Keywords
Monolith NT.115; Protein-Small Molecule; Biomolecular Interaction
Receptor binding by a ferret-transmissible H5 avian influenza virus
Nature
Xiaoli Xiong, Peter J. Coombs, Stephen R. Martin, Junfeng Liu, Haixia Xiao, John W. McCauley, Kathrin Locher, Philip A. Walker, Patrick J. Collins, Yoshihiro Kawaoka, John J. Skehel & Steven J. Gamblin
Abstract
Cell-surface-receptor binding by influenza viruses is a key determinant of their transmissibility, both from avian and animal species to humans as well as from human to human. Highly pathogenic avian H5N1 viruses that are a threat to public health have been observed to acquire affinity for human receptors, and transmissible-mutant-selection experiments have identified a virus that is transmissible in ferrets1, 2, 3, the generally accepted experimental model for influenza in humans. Here, our quantitative biophysical measurements of the receptor-binding properties of haemagglutinin (HA) from the transmissible mutant indicate a small increase in affinity for human receptor and a marked decrease in affinity for avian receptor. From analysis of virus and HA binding data we have derived an algorithm that predicts virus avidity from the affinity of individual HA-receptor interactions. It reveals that the transmissible-mutant virus has a 200-fold preference for binding human over avian receptors. The crystal structure of the transmissible-mutant HA in complex with receptor analogues shows that it has acquired the ability to bind human receptor in the same folded-back conformation as seen for HA from the 1918, 1957 (ref. 4), 1968 (ref. 5) and 2009 (ref. 6) pandemic viruses. This binding mode is substantially different from that by which non-transmissible wild-type H5 virus HA binds human receptor. The structure of the complex also explains how the change in preference from avian to human receptors arises from the Gln226Leu substitution, which facilitates binding to human receptor but restricts binding to avian receptor. Both features probably contribute to the acquisition of transmissibility by this mutant virus.
Keywords
Animals; Birds/metabolism/virology; Chick Embryo; Crystallography, X-Ray; Ferrets/*virology; Hemagglutinin Glycoproteins; Influenza Virus/*chemistry/genetics/*metabolism; *Host Specificity;
Humans; Influenza A Virus; H5N1 Subtype/chemistry/*genetics/*metabolism/pathogenicity; Models, Biological; Models, Molecular; Mutation; Orthomyxoviridae Infections/*transmission/*virology; Protein Conformation; Receptors, Virus/*metabolism; Species Specificity; Monolith NT.115; Protein-Protein

 More than binding affinities
More than binding affinities