Abstract: Hypertrophic cardiomyopathy (HCM) is primarily caused by mutations in β-cardiac myosin and myosin-binding protein-C (MyBP-C). Changes in the contractile parameters of myosin measured so far do not explain the clinical hypercontractility caused by such mutations. We propose that hypercontractility is due to an increase in the number of myosin heads (S1) that are accessible for force production. In support of this hypothesis, we demonstrate myosin tail (S2)-dependent functional regulation of actin-activated human β-cardiac myosin ATPase. In addition, we show that both S2 and MyBP-C bind to S1 and that phosphorylation of either S1 or MyBP-C weakens these interactions. Importantly, the S1-S2 interaction is also weakened by four myosin HCM-causing mutations but not by two other mutations. To explain these experimental results, we propose a working structural model involving multiple interactions, including those with myosin's own S2 and MyBP-C, that hold myosin in a sequestered state.
Keywords
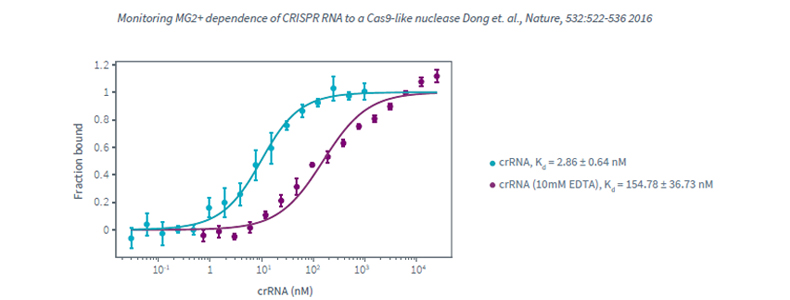
Monolith NT.115Pico, Protein-Nucleic acids interaction, high affinity interaction, Biomolecular Interaction
Orthogonal Methods for Characterizing the Unfolding of Therapeutic Monoclonal Antibodies: Differential Scanning Calorimetry, Isothermal Chemical Denaturation, and Intrinsic Fluorescence with Concomitant Static Light Scattering
Methods in Enzymology
Deniz B. Temel, Pavel Landsman, Mark L. Brader
Abstract
Evaluating prospective protein pharmaceutical stability from accelerated screening is a critical challenge in biotherapeutic discovery and development. Measurements of protein unfolding transitions are widely employed for comparing candidate molecules and formulations; however, the interrelationships between intrinsic protein conformational stability and pharmaceutical robustness are complex and thermal unfolding measurements can be misleading. Beyond the discovery phase of drug development, astute formulation design is one of the most crucial factors enabling the protein to resist damage to its higher order structure-initially from bioprocessing stresses, then from stresses encountered during its journey from the product manufacturing site to the bloodstream of the patient. Therapeutic monoclonal antibodies are multidomain proteins that represent a large and growing segment of the biotechnology pipeline. In this chapter, we describe how differential scanning calorimetry may be leveraged synergistically with isothermal chemical denaturation and intrinsic fluorescence with concomitant static light scattering to elucidate characteristics of mAb unfolding and aggregation that are helpful toward understanding and designing optimal pharmaceutical compositions for these molecules.
Pawel Linke, Kwame Amaning, Melanie Maschberger, Francois Vallee, Valerie Steier, Philipp Baaske, Stefan Duhr, Dennis Breitsprecher, Alexey Rak
DNA-binding proteins from marine bacteria expand the known sequence diversity of TALE-like repeats
Nucleic Acids Research
Orlando de Lange, Christina Wolf, Philipp Thiel, Jens Krüger, Christian Kleusch,Oliver Kohlbacher, Thomas Lahaye
Abstract
Transcription Activator-Like Effectors (TALEs) of Xanthomonas bacteria are programmable DNA binding proteins with unprecedented target specificity. Comparative studies into TALE repeat structure and function are hindered by the limited sequence variation among TALE repeats. More sequence-diverse TALE-like proteins are known from Ralstonia solanacearum (RipTALs) and Burkholderia rhizoxinica (Bats), but RipTAL and Bat repeats are conserved with those of TALEs around the DNA-binding residue. We study two novel marine-organism TALE-like proteins (MOrTL1 and MOrTL2), the first to date of non-terrestrial origin. We have assessed their DNA-binding properties and modelled repeat structures. We found that repeats from these proteins mediate sequence specific DNA binding conforming to the TALE code, despite low sequence similarity to TALE repeats, and with novel residues around the BSR. However, MOrTL1 repeats show greater sequence discriminating power than MOrTL2 repeats. Sequence alignments show that there are only three residues conserved between repeats of all TALE-like proteins including the two new additions. This conserved motif could prove useful as an identifier for future TALE-likes. Additionally, comparing MOrTL repeats with those of other TALE-likes suggests a common evolutionary origin for the TALEs, RipTALs and Bats.
Keywords
Monolith NT.115, Protein-Nucleic Acids, Biomolecular Interaction, Prometheus NT.48, Protein Stability
Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling
Science
Abstract
Cell division progresses to anaphase only after all chromosomes are connected to spindle microtubules through kinetochores and the spindle assembly checkpoint (SAC) is satisfied. We show that the amino-terminal localization module of the SAC protein kinase MPS1 (monopolar spindle 1) directly interacts with the HEC1 (highly expressed in cancer 1) calponin homology domain in the NDC80 (nuclear division cycle 80) kinetochore complex in vitro, in a phosphorylation-dependent manner. Microtubule polymers disrupted this interaction. In cells, MPS1 binding to kinetochores or to ectopic NDC80 complexes was prevented by end-on microtubule attachment, independent of known kinetochore protein-removal mechanisms. Competition for kinetochore binding between SAC proteins and microtubules provides a direct and perhaps evolutionarily conserved way to detect a properly organized spindle ready for cell division.
Keywords:
Monolith NT.115, Protein-Protein, Multiple Component Interactions, Biomolecular Interaction
CELL DIVISION CYCLE. Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C
Science
Abstract
The spindle checkpoint of the cell division cycle senses kinetochores that are not attached to microtubules and prevents precocious onset of anaphase, which can lead to aneuploidy. The nuclear division cycle 80 complex (Ndc80C) is a major microtubule receptor at the kinetochore. Ndc80C also mediates the kinetochore recruitment of checkpoint proteins. We found that the checkpoint protein kinase monopolar spindle 1 (Mps1) directly bound to Ndc80C through two independent interactions. Both interactions involved the microtubule-binding surfaces of Ndc80C and were directly inhibited in the presence of microtubules. Elimination of one such interaction in human cells caused checkpoint defects expected from a failure to detect unattached kinetochores. Competition between Mps1 and microtubules for Ndc80C binding thus constitutes a direct mechanism for the detection of unattached kinetochores.
Keywords: Monolith NT.115, Protein-Protein, Biomolecular Interaction
VCAM-1 directed target-sensitive liposomes carrying CCR2 antagonists bind to activated endothelium and reduce adhesion and transmigration of monocytes
European Journal of Pharmaceutics and Biopharmaceutics
Manuela Calin, Daniela Stan, Martin Schlesinger, Viorel Simion, Mariana Deleanu, Cristina Ana Constantinescu, Ana-Maria Gan, Monica Madalina Pirvulescu, Elena Butoi, Ileana Manduteanu, Marian Bota, Marius Enachescu, Lubor Borsig, Gerd Bendas, Maya Simionescu
Abstract
Chemokines are critically involved in the development of chronic inflammatory-associated diseases such as atherosclerosis. We hypothesized that targeted delivery of compounds to the surface of activated endothelial cells (EC) interferes with chemokine/receptor interaction and thereby efficiently blocks inflammation. We developed PEGylated target-sensitive liposomes (TSL) encapsulating a CCR2 antagonist (Teijin compound 1) coupled with a specific peptide recognized by endothelial VCAM-1 (Vp-TSL-Tj). TSL were characterized for size (by dynamic light scattering), the amount of peptide coupled at the liposomal surface and Teijin release (by HPLC). We report that Vp-TSL-Tj binds specifically to activated EC in vitro and in situ, release the entrapped Teijin and prevent the transmigration of monocytes through activated EC. This is the first evidence that nanocarriers which transport and release chemokine inhibitors at specific pathological sites can reduce chemokine-dependent inflammatory processes.
Keywords: CCR2 antagonist; Endothelium; Monocyte; Target-sensitive liposomes; Targeted drug delivery; VCAM-1
Surface acoustic wave biosensor as a functional quality method in pharmaceutics
Sensors and Actuators B: Chemical
Nathalie Bracke, Sophia Barhdadi, Evelien Wynendaele, Bert Gevaert, Matthias D’Hondt, Bart De Spiegeleer
Abstract
With the recent successes of biopharmaceuticals, new bioanalytical tools are needed for the functional quality characterization of new biological entities and biosimilars towards marketing authorization approval and during the product life. Biosensors are emerging technologies in the pharmaceutical field in drug discovery, development and Quality Control (QC) stages; however, they are not yet included in any pharmacopoeia. Surface acoustic wave (SAW) biosensors allow the user to detect and quantify binding events, giving information not only on affinity (KD) and kinetics (kon and koff), but also on viscoelastic effects. We present here an analytical quality by design (aQbD) based approach using somatropin (rhGH, recombinant human growth hormone) and derivatives as model biotechnological drugs and an antibody interaction partner to evaluate the functional quality. Using techniques described in ICH Q2, Q5 and Q8-11, we evaluated the performance of this SAW biosensor technique from a pharmaceutical quality point of view. CMAs (Critical Method Attributes) such as sensitivity, selectivity and variability are evaluated as a function of different experimental variables using DoEs (design of experiments). We suggest qualification tests as well as SSTs (system suitability tests), as required by the pharmaceutical competent authorities and recommend the inclusion of biosensor techniques into the different pharmacopoeia and pharmaceutical guidelines.
Keywords: Surface acoustic wave biosensor; Analytical quality by design; (Modified) somatropin; Pharmaceutical GLP/GMP; Biosensor method development
